Abstract
The fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) is the most common recurrent mutation in acute myeloid leukemia (AML). FLT3-ITD varies in size from 3 to over 200 bp, resulting in elongation of juxtamembrane domain coded by exon 14 and constitutive kinase activation. The FLT3-ITD is a poor prognostic marker found in 20-30% of AML. However, molecular mechanisms underlying ITD formation are remained to be elucidated. We have analyzed FLT3-ITD mutation-positive AML cases using next-generation sequencing (NGS) and speculated that DNA breakage initiates ITD formation. We developed artificial FLT3-ITD formation assay using CRISPR/Cas9 system.
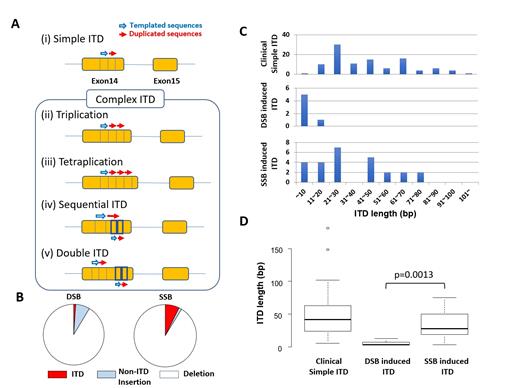
First, genomic DNA from 25 cases with FLT3-ITD mutation-positive AML was used to PCR-amplify the ITD cluster region (ICR; FLT3 exon 14-15) and sequenced by NGS. We extracted more than 3 bp of deletion and insertion. Total 139 independent ITD sequences were identified at varied variant allele frequency (VAF) (0.0005%-45.7%). Each case had 1 to 13 ITDs (median 4 clones). The length of ITDs was 6 to 201 bp (median 48 bp) and 135 (97.1%) unique ITDs showed length with a multiple of 3 bp. In addition, we found 32 clones with Complex ITD, which is considered to have multiple ITD events. Simple FLT3-ITD showed consecutive two repeated sequences with or without filler sequence between the repeated sequences (Fig. Ai). However, some clones showed three or four repetitive sequences (Fig. Aii, Aiii). Furthermore, some clones had sequential second ITD including parts of the first ITD (Fig. Aiv), and some clones had two ITDs at adjacent or distant locations (Fig. Av). These "Complex ITD" was seen in 18 (72%) cases and always accompanying originated "Simple ITD" clones with higher VAF. Total 59 independent deletion sequences were identified in 24 out of 25 (96%) cases. Length of deletion of ICR is 3 to 204 bp (median 4.5 bp). Deletion clone is always rare clone which had a few reads. Non-ITD insertions were found only in 5 clones. The presence of multiple ITD clones in a single case, Complex ITD clones, and deletion clones in the ICR suggest that the ICR is prone to genomic damage, and the mutation process is ongoing in each AML case creating various ITD/deletions.
Based on the observation of clinical samples, we investigated whether artificially induced DNA break at ICR repaired as ITDs in the human cell line. The FLT3 ICR was TA-cloned into the pGEM-T easy vector. FLT3 exon 14-targeted guide RNA and Cas9 protein were incubated with the vector in vitro and transfected to HEK293T cells. We compared conventional Cas9 inducing double-strand break (DSB) and Cas9-nickase inducing single-strand break (SSB) to determine the efficacy of ITD formation depending on the different DNA break modes. Genomic DNA was extracted from transfected HEK293T and successfully repaired ICR was amplified with primers annealing to pGEM-T easy vector flanking the cloning site. The amplified PCR product was analyzed by NGS with a 250 bp pair-end read. We extracted 545 and 353 miss repair events from DSB and SSB experiments respectively. The DSB of ICR was repaired as ITDs 1.1%, non-ITD insertions 7.5%, and deletions 91.4% (Fig. B). On the other hand, SSB of ICR was repaired as ITDs 7.3%, non-ITD insertions 1.4%, and deletions 91.2% (Fig. B). Within insertion event, ITD frequency was significantly higher in SSB compared to DSB. (p<0.001; chi-square test). Length of ITDs were 3 to 13 bp (median 3.5 bp) in DSB and 3 to 75 bp (median 28 bp) in SSB experiment (Fig. C). The ITD formed by the SSB was significantly longer than that formed by the DSB (p=0.0013; Mann-Whitney U test) and similar to observation in clinical simple ITD (Fig. C, D). Furthermore, we induced in-vivo SSB at endogenous FLT3 exon14 in HEK293T cells and successfully detected in situ ITDs. Using CRISPR induced SSB, we might develop a cell line with artificial FLT3-ITD which would contribute to deepen understanding of FLT3 biology.
SSBs at FLT3 ICR could be a key initiator of FLT3-ITD formation. Progress in understanding the molecular mechanism of FLT3-ITD formation may lead to the development of therapeutic agents in the future.
Nakagawa: AbbVie GK: Research Funding; Takeda Pharmaceutical Company: Research Funding. Kondo: Astellas Pharma Inc.: Consultancy, Honoraria; Sanwa Kagaku Kenkyusho CO.,LTD: Consultancy; Sumitomo Dainippon Pharma: Honoraria; Bristol-Myers Squibb Company: Honoraria; Novartis Pharma KK: Honoraria; Otsuka Pharmaceutical: Consultancy, Honoraria, Research Funding; Abbvie: Honoraria; Pfizer: Honoraria. Teshima: Astellas Pharma Inc.: Research Funding; Takeda Pharmaceutical Company: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin Co.,Ltd.: Honoraria, Research Funding; Merck Sharp & Dohme: Membership on an entity's Board of Directors or advisory committees; Fuji pharma CO.,Ltd: Research Funding; Pfizer Inc.: Honoraria; TEIJIN PHARMA Limited: Research Funding; Gentium/Jazz Pharmaceuticals: Consultancy; Novartis International AG: Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding; Janssen Pharmaceutical K.K.: Other; Bristol Myers Squibb: Honoraria; CHUGAI PHARMACEUTICAL CO., LTD.: Research Funding; Sanofi S.A.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal